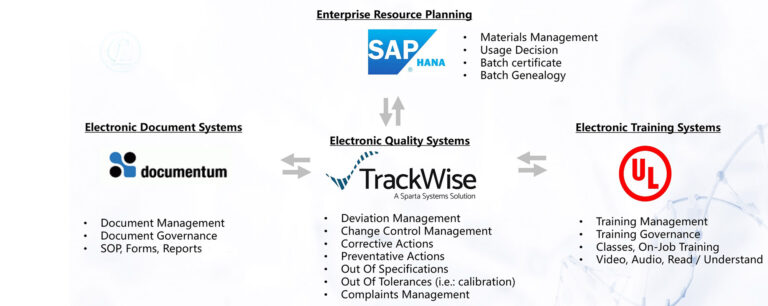
September 22, 2022, Shenzhen – CL Biologics (CLB) announces today at the CLB Watson Center for Life Sciences and Technology the launch of its digital Quality Management Suites (eQMS), integrating three key quality systems: TrackWise®, Documentum® and ComplianceWire®.
Under the leadership of Mr. Michael Garvey, President and Managing Director, and Mr. Kartik Padh, Chief Quality Officer, the CLB quality team worked closely with vendor Tri-I Biotech (Shanghai) and implemented the eQMS in 6 months, which went live on September 16, 2022.
With the eQMS in place, CLB not only moves away from paper-based management but also creates an ecosystem to securely share data. Data generated in real time can be managed whilst simultaneously ensuring data integrity for customers.
Mr. Kartik Padh remarked, “The ability to share data and information real time from quality systems is phenomenal.” He emphasized that quality is CLB’s lifeline and CLB strives to meet its clients’ highest demands in quality and data integrity.
Mr. Michael Garvey pointed out that CLB is aiming to become a leader in the Biomanufacturing 4.0 revolution. In the post-COVID-19 world, it is imperative for the biopharma industry to scale faster and more effectively, in order to meet increasing demands for life-saving therapeutics and vaccines. CLB as “the World’s Local CDMO” can help our biopharma clients to achieve these goals. The eQMS are only the beginning of CLB’s efforts to continuously implement and integrate smart digital systems, and keep improving process design.